This is no small feet! A nutritional intervention performing this well in phase 3 clinical cancer trials deserves some recognition.
Second to Magnesium, Vitamin B3 is my favorite nutraceutical, treating anything and everything from acne to anxiety. This clinical trial shows the amazing benefits of nicotinamide on the prevention of squamous cell and basal cell carcinomas.
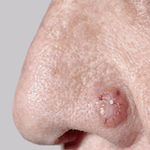
Although these types of skin cancers are not deadly, they certainly plague people in places that are not desirable. Squamous cell and basal cell cancers typically occur where sun exposure is the greatest …. right on the face!
At the clinic we use the cryoprobe (high pressure liquid nitrogen) to remove these lesions from peoples sensitive areas. The problem is, as many people that battle squamous cell or basal cell carcinomas know, the recurrence rates for these skin lesions are huge.
Nicotinamide seems to offer the prevention that people need, and if it doesn’t work, at the very least you will be a lot calmer and likely sleep a lot better by the end of the trial!
It’s a little hard to find in the health food stores, so if you are thinking about giving it a try make sure to get the right stuff!
Chen AC, Martin AJ, Choy B, et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N Engl J Med. 2015 Oct 22;373(17):1618-1626. (Original) PMID: 26488693
Background Nonmelanoma skin cancers, such as basal-cell carcinoma and squamous-cell carcinoma, are common cancers that are caused principally by ultraviolet (UV) radiation. Nicotinamide (vitamin B3) has been shown to have protective effects against damage caused by UV radiation and to reduce the rate of new premalignant actinic keratoses. Methods In this phase 3, double-blind, randomized, controlled trial, we randomly assigned, in a 1:1 ratio, 386 participants who had had at least two nonmelanoma skin cancers in the previous 5 years to receive 500 mg of nicotinamide twice daily or placebo for 12 months. Participants were evaluated by dermatologists at 3-month intervals for 18 months. The primary end point was the number of new nonmelanoma skin cancers (i.e., basal-cell carcinomas plus squamous-cell carcinomas) during the 12-month intervention period. Secondary end points included the number of new squamous-cell carcinomas and basal-cell carcinomas and the number of actinic keratoses during the 12-month intervention period, the number of nonmelanoma skin cancers in the 6-month postintervention period, and the safety of nicotinamide. Results At 12 months, the rate of new nonmelanoma skin cancers was lower by 23% (95% confidence interval [CI], 4 to 38) in the nicotinamide group than in the placebo group (P=0.02). Similar differences were found between the nicotinamide group and the placebo group with respect to new basal-cell carcinomas (20% [95% CI, -6 to 39] lower rate with nicotinamide, P=0.12) and new squamous-cell carcinomas (30% [95% CI, 0 to 51] lower rate, P=0.05). The number of actinic keratoses was 11% lower in the nicotinamide group than in the placebo group at 3 months (P=0.01), 14% lower at 6 months (P<0.001), 20% lower at 9 months (P<0.001), and 13% lower at 12 months (P=0.001). No noteworthy between-group differences were found with respect to the number or types of adverse events during the 12-month intervention period, and there was no evidence of benefit after nicotinamide was discontinued. Conclusions Oral nicotinamide was safe and effective in reducing the rates of new nonmelanoma skin cancers and actinic keratoses in high-risk patients. (Funded by the National Health and Medical Research Council; ONTRAC Australian New Zealand Clinical Trials Registry number, ACTRN12612000625875 .).